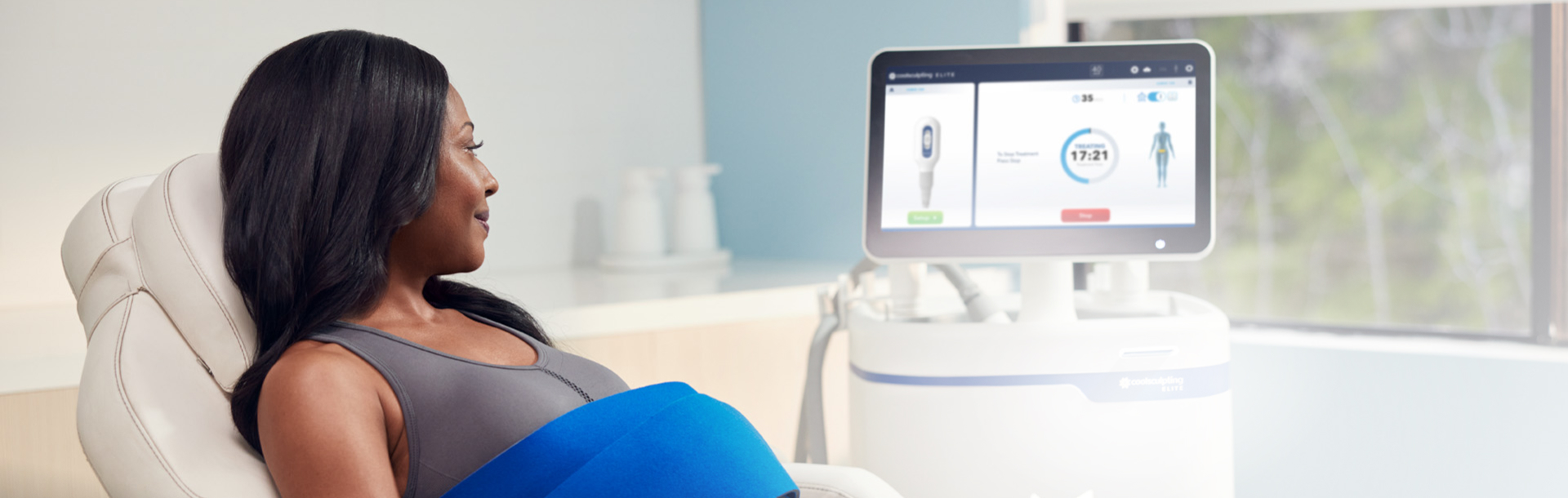
Why does fat freezing work?
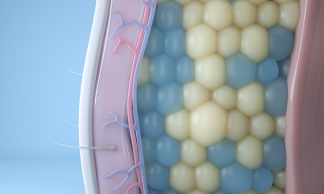
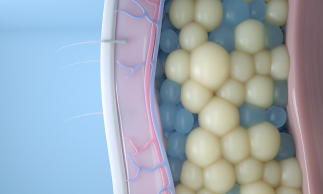
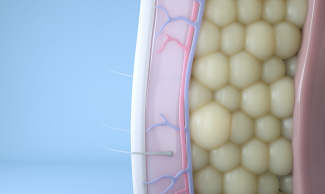
After observing dimples in children who ate cold popsicles, renowned Harvard University scientists Dieter Manstein, MD, PhD, and R. Rox Anderson, MD, developed a unique cooling process that could selectively target and kill fat cells. That fat-freezing technology, or cryolipolysis, became the science behind CoolSculpting.
Today, our cryolipolysis treatment is one-of-a-kind in its ability to induce apoptosis in fat cells, by using cold to gently and gradually reduce the fat layer. Cryolipolysis selectively freezes treated fat cells to a specific temperature, damaging the fat cells without causing harm to the surrounding tissue. Treated fat cells die and are subsequently eliminated from the body. Patients can see up to 20-25% fat reduction in treated areas.1 2 Results may be seen as early as 1-3 months after treatment.
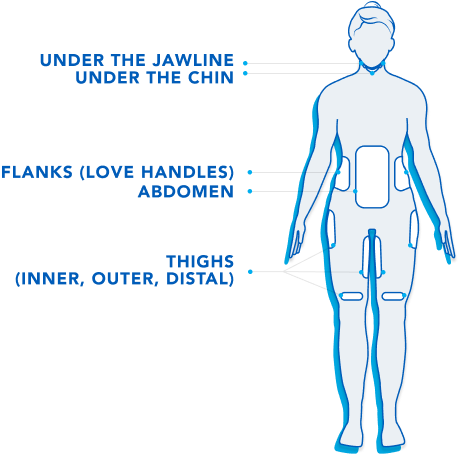
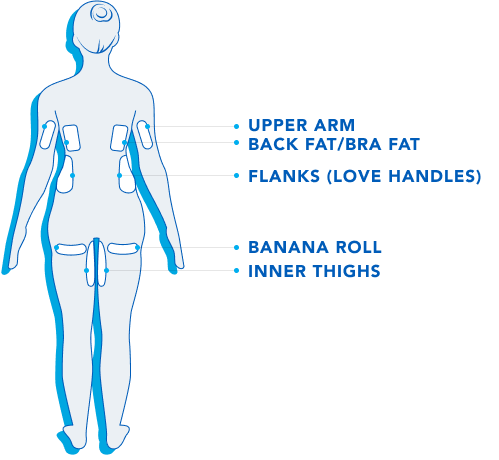
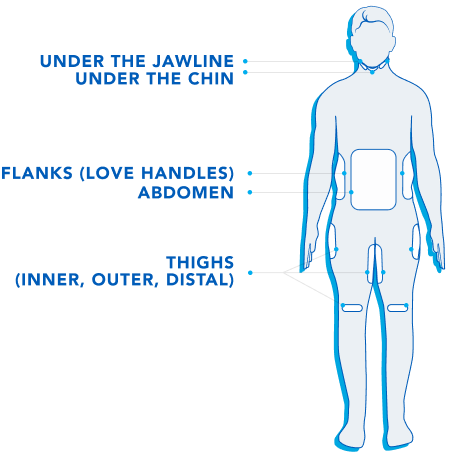
Treatment Areas.
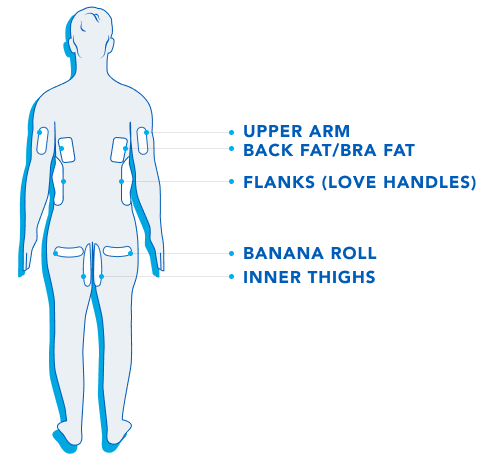
CoolSculpting® is FDA-cleared to treat 9 different areas of the body: Visible bulges under the chin and jawline areas, thighs, abdomen and flanks, along with bra fat, back fat, underneath the buttocks, and upper arms.
Want to learn more?
Internal Error
CoolSculpting® and CoolSculpting® Elite Indications
CoolSculpting® and CoolSculpting® Elite are FDA-cleared for the treatment of visible fat bulges in the thigh, abdomen, and flank, along with bra fat, back fat, underneath the buttocks (also known as banana roll), and upper arm in patients with a Body Mass Index (BMI) of ≤ 30 and in submental and submandibular areas in patients with a BMI of ≤ 46.2. It is also FDA-cleared to affect the appearance of lax tissue with submental area treatments.
CoolSculpting® and CoolSculpting® Elite Important Safety Information
CoolSculpting® and CoolSculpting® Elite are contraindicated in patients with cryoglobulinemia, cold agglutinin disease, or paroxysmal cold hemoglobinuria.
Ask your patient about any medical conditions including recent surgery, pre-existing hernia, and any known sensitivities or allergies.
During the procedure patients may experience sensations of pulling, tugging, mild pinching, intense cold, tingling, stinging, aching, and cramping at the treatment site. These sensations subside as the area becomes numb. Following the procedure, typical side effects include temporary redness, swelling, blanching, bruising, firmness, tingling, stinging, tenderness, cramping, aching, itching, or skin sensitivity, and sensation of fullness in the back of the throat after submental or submandibular area treatment.
Rare side effects may also occur. Paradoxical hyperplasia (visibly enlarged tissue volume in the treated area) may develop 2 to 5 months after treatment, will not resolve on its own, and may require surgical intervention for correction.
As with any medical procedure, a consultation should be done by a licensed healthcare professional to determine if the patient is a candidate for treatment. For a complete list of Contraindications, Warnings, Precautions, and Potential Side Effects, consult the CoolSculpting® System User Manual and the CoolSculpting® Elite System User Manual. Treatment applications that deviate from the guidelines are not recommended.
References: 1. Coleman SR, Sachdeva K, Egbert BM, Preciado J, Allison J. Clinical efficacy of noninvasive cryolipolysis and its effects on peripheral nerves. Aesthetic Plast Surg. 2009;33(4):482-488 2. Boey GE, Wasilenchuk JL. Enhanced clinical outcomes with manual massage following cryolipolysis treatment: a 4-month study of safety and efficacy. Lasers Surg Med. 2014;46(1):20-26. 3. Bernstein EF. Longitudinal Evaluation of cryolipolysis Efficacy. J Cosmet Dermatol. 4. Zelickson B, Egbert BM, Preciado J, et al. Cryolipolysis for Noninvasive Fat Cell Destruction: Initial Results From a Pig Model. Dermatol Surg. 5. Nelson AA, Wasserman D, Avram MM. Cryolipolysis for the Reduction of Excess Adipose Tissue. Semin Cutan Med Surg. 6. Bernstein EF. Longitudinal Evaluation of cryolipolysis Efficacy. J Cosmet Dermatol. 7. Stevens WG, Pietrzak LK, Spring MA. Broad Overview of a Clinical and Commercial Experience With CoolSculpting. Aesthetic Surg J. 8. Dierickx CC, Mazer JM, Sand M, et al. Safety, Tolerance, and Patient Satisfaction With Noninvasive cryolipolysis. Dermatol Surg. 9. Coleman SR, Sachdeva K, Egbert BM. Clinical Efficacy of Noninvasive cryolipolysis and Its Effects on Peripheral Nerves. Aesthetic Plast Surg. 10. Klein KB, Zelickson B, Ropelle JG, et al. Noninvasive cryolipolysis for Subcutaneous Fat Reduction Does Not Affect Serum Lipid Levels or Liver Function Tests. Lasers Surg Med.