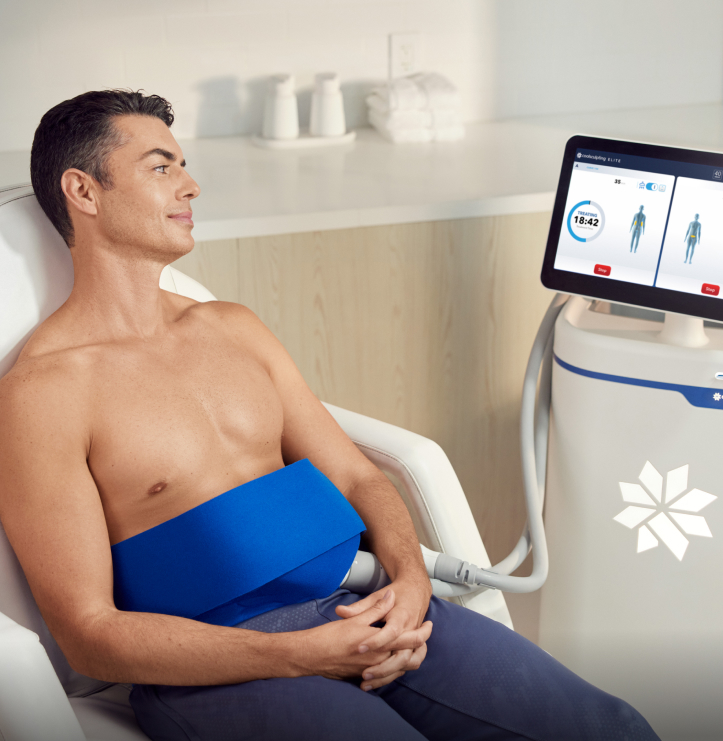
You Inspired Us To Think Deeper, Push Further, and Engineer Smarter.
We’ve transformed the CoolSculpting® experience for both providers and patients. Our most sophisticated system yet, CoolSculpting Elite features dual applicators, an advanced touchscreen, and a lighter, more compact build—all designed to help take your practice further.
Our Technology. Your Opportunity.
Your success requires more than just the technology you offer. That’s why our team of representatives offers custom reports to illustrate the consumer demand and revenue opportunity when your practice adds CoolSculpting Elite to your services.
Every Efficiency Counts.
The Elite system streamlines the entire treatment process for your practice.
Increase Productivity
Dual applicators allow you to complete more treatments in the same amount of time.*
Easier Storage
Detachable applicators eliminate the need for extra equipment that takes up space.
Streamlined Card System
Universal cards can operate any applicator for either dual or solo treatments.
*As compared to using one applicator.

Every Contour Counts.
We completely reengineered our applicators to optimize patient treatments.
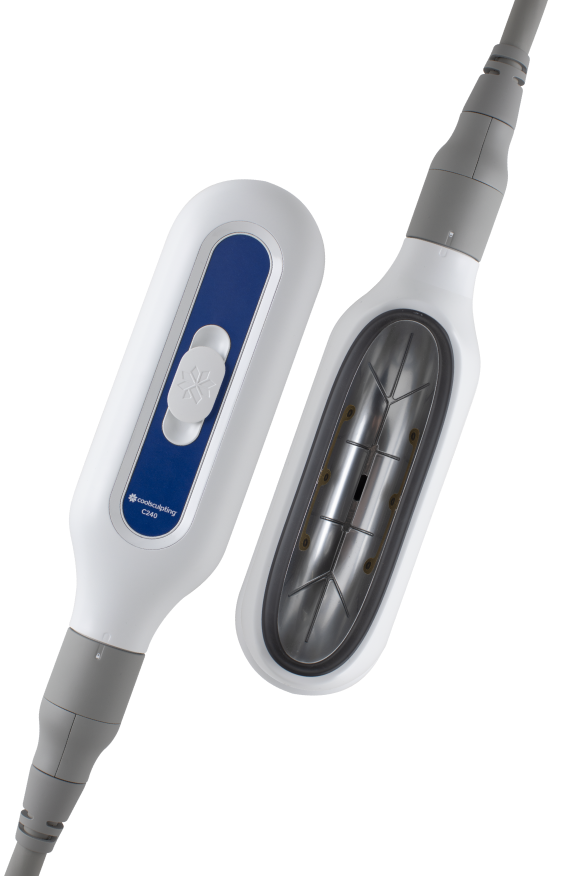
Redesigned C-Shaped Cup
Expertly designed for better fit and function to complement a patient's natural curves.†
Larger Cooling Area
Optimized for up to an 18% larger cooling area.1,2,‡,§
† In an engineering study evaluating the fit and comfort of CoolSculpting® Elite and CoolAdvantage™ applicator cups, 41 patients underwent a total of 665 tissue draws. Fit was determined by clinician-reported results on tissue contact and vacuum seal.
‡ Up to 18% larger surface area with Elite Curve 240™ applicator as compared to Cool Advantage Plus™ applicator.
§ The clinical significance of these data has not been established.
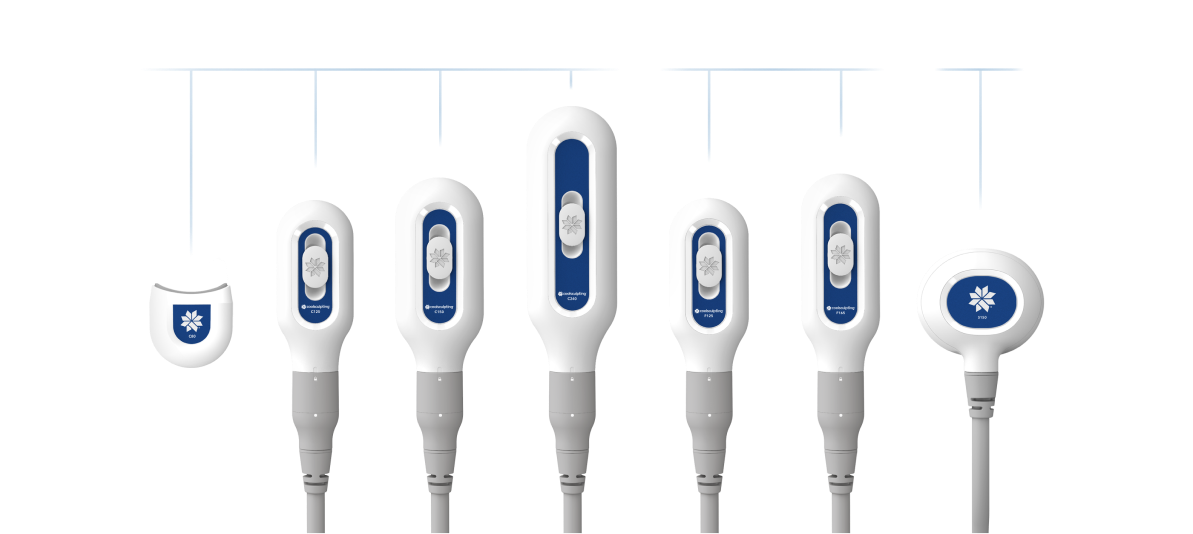
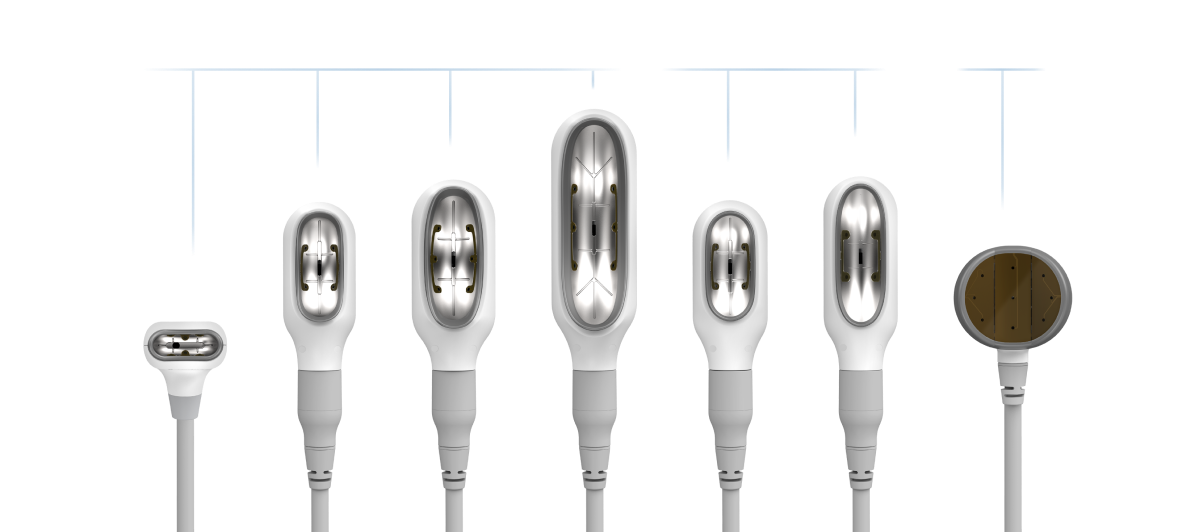
Every treatment counts.
The Elite applicator collection, which includes seven different sizes, allows you to personalize each patient’s plan and treat a variety of stubborn fat areas. Each treatment session helps take your patients one step further - with most reaching their body contouring goals after just two sessions.
Ready for the elite experience?
Internal Error
CoolSculpting® and CoolSculpting® Elite Indications
CoolSculpting® and CoolSculpting® Elite are FDA-cleared for the treatment of visible fat bulges in the thigh, abdomen, and flank, along with bra fat, back fat, underneath the buttocks (also known as banana roll), and upper arm in patients with a Body Mass Index (BMI) of ≤ 30 and in submental and submandibular areas in patients with a BMI of ≤ 46.2. It is also FDA-cleared to affect the appearance of lax tissue with submental area treatments.
CoolSculpting® and CoolSculpting® Elite Important Safety Information
CoolSculpting® and CoolSculpting® Elite are contraindicated in patients with cryoglobulinemia, cold agglutinin disease, or paroxysmal cold hemoglobinuria.
Ask your patient about any medical conditions including recent surgery, pre-existing hernia, and any known sensitivities or allergies.
During the procedure patients may experience sensations of pulling, tugging, mild pinching, intense cold, tingling, stinging, aching, and cramping at the treatment site. These sensations subside as the area becomes numb. Following the procedure, typical side effects include temporary redness, swelling, blanching, bruising, firmness, tingling, stinging, tenderness, cramping, aching, itching, or skin sensitivity, and sensation of fullness in the back of the throat after submental or submandibular area treatment.
Rare side effects may also occur. Paradoxical hyperplasia (visibly enlarged tissue volume in the treated area) may develop 2 to 5 months after treatment, will not resolve on its own, and may require surgical intervention for correction.
As with any medical procedure, a consultation should be done by a licensed healthcare professional to determine if the patient is a candidate for treatment. For a complete list of Contraindications, Warnings, Precautions, and Potential Side Effects, consult the CoolSculpting® System User Manual and the CoolSculpting® Elite System User Manual. Treatment applications that deviate from the guidelines are not recommended.
References: 1. Data on file, Allergan, 2020; V3 Applicator Specifications for Regulatory Submissions (CS-305044-A). 2. Data on file, Allergan; Applicator Specifications for Regulatory Submissions (DR17489-K). 3. Data on file, Allergan, July 2019; 8MM CoolSculpting Treatments.